Adsorption principle of zeolite molecular sieve
2025-04-01
Honeycomb zeolite molecular sieves have a unique adsorption principle, mainly based on the following aspects:
Pore size sieving effect Honeycomb zeolite molecular sieves have uniform and small pore sizes, which are comparable to the sizes of many molecules. When molecules in a mixture approach the zeolite molecular sieve, only those molecules smaller than the pore size can enter the channels, while larger molecules are blocked, thus achieving the sieving of molecules of different sizes.
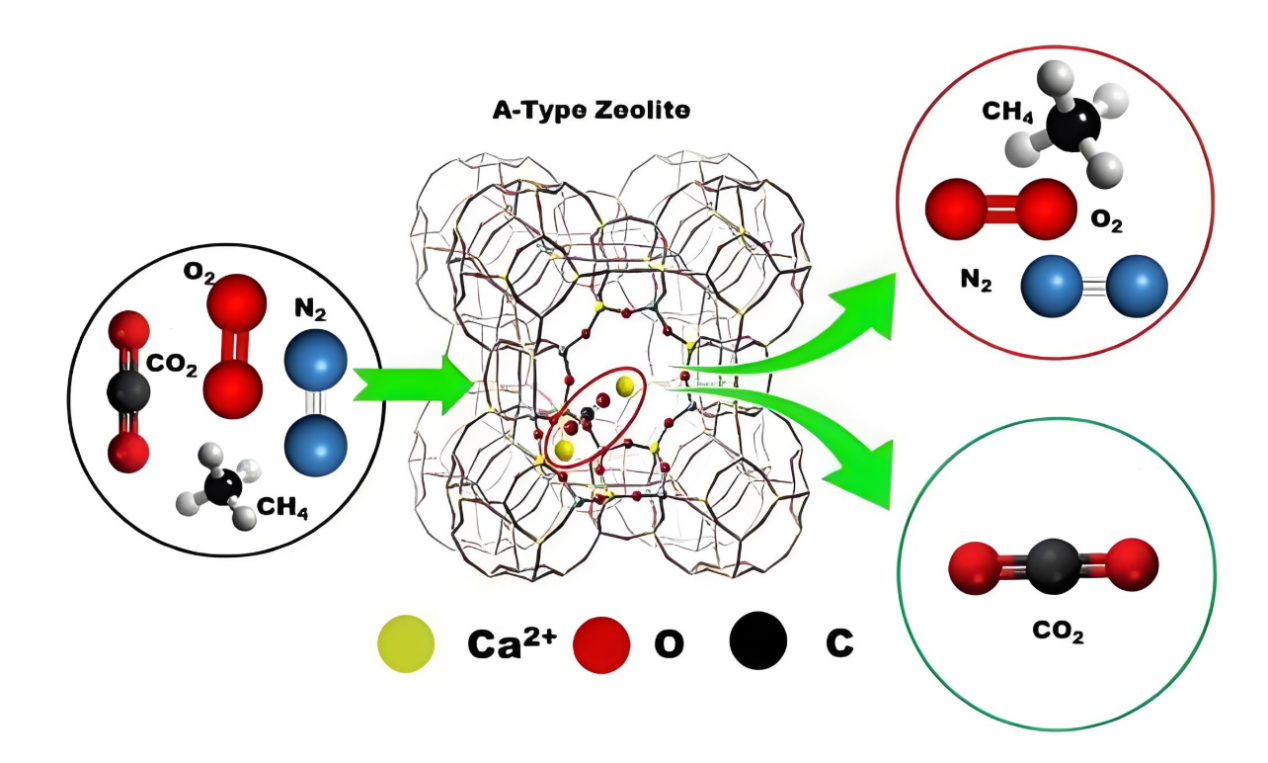
Electrostatic attraction: The aluminosilicate structure in the zeolite molecular sieve framework generates a certain electrostatic field. Due to the different electronegativity of silicon (Si) and aluminum (Al) atoms, the aluminum oxygen tetrahedron carries a negative charge. To maintain electrical neutrality, some cations (such as Na+, K+, Ca2+ etc.) exist in the channels. These cations can generate electrostatic attraction with polar or polarizable molecules, making it easier for molecules to be adsorbed on the inner surface of the channels.
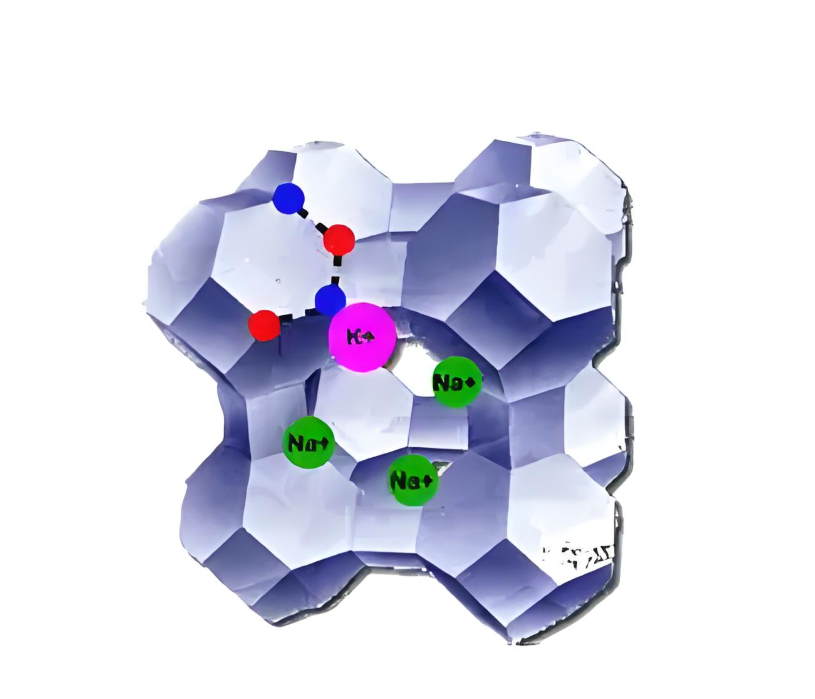
Surface adsorption Honeycomb zeolite molecular sieves have a large specific surface area, and the inner channel surface has high surface energy. When molecules approach the channel surface, they are adsorbed on the surface due to the influence of van der Waals forces and other forces. This surface adsorption has a certain effect on various molecules, especially for those molecules that can form hydrogen bonds or other weak interactions with the channel surface, the adsorption effect is more obvious.
Previous Page
Previous Page:
Recommended News
Basic principles of molecular sieves
2025-06-13
Types and Selection of Molecular Sieves
2025-06-13
Application cases of molecular sieves
2025-06-13









